
Research report – July 2008
Former lead researcher, Denise Caruso, provided the following progress report.
Active Immunotherapy for Children with Brain and Solid Tumours
Despite the relative successes in treating childhood cancers in recent years there remain very few effective treatment options for patients who fail current therapies. Additionally, current therapies that include chemotherapy and radiotherapy have reached the limit of toxicity that patients can withstand. Children with cancer must deal with both the terrible effects of their disease and also with the harshness of their treatments. At present, current aggressive therapies are the only options available to relapsed patients and patients with highly aggressive tumours such as glioblastoma and neuroblastoma, and will in most cases ultimately fail. Therefore it is necessary to explore new directions for treating tumours in children that are effective and safe.
Immunotherapy is an option for treatment for these children that is still in the clinical trial stage, but has the potential of being an effective and well tolerated treatment by these patients. The immune system is very powerful and very specific, and therefore makes a desirable system to harness for anti-cancer therapy. While the potential for this kind of therapy for children is great there are still several issues to be resolved to optimise the treatment regimen for maximum efficacy. Our group has previously conducted Phase I clinical trials using patient tumour material to educate their immune systems to fight their own tumours, thereby producing a tailor-made therapy for each child specific to their particular cancer. While we were able to show that immunotherapy in children with cancer was both feasible and safe, and had no side effects, the anti-tumour effects were minimal. Currently there are now improved techniques and protocols that should maintain safety and feasibility standards but that will also have more effective anti-tumour activity. These protocols are now ready for phase I clinical trials in children with solid and brain tumours.
Primary Aims of proposed immunotherapy protocol:
1. Demonstrate its safety in children with cancer
2. Demonstrate its feasibility in children with cancer
Secondary Aims of proposed immunotherapy protocol:
1. Assess the anti-tumour responses by traditional clinical measures
2. Assess the stimulation and effectiveness of the immune system before and after treatment by this immunotherapy protocol.
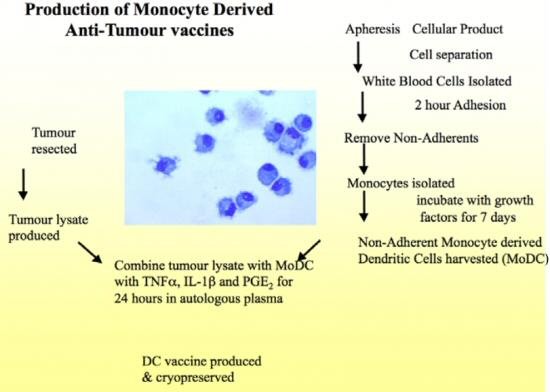
Progress to date.
We have recruited ten patients to the trial: five newly diagnosed neuroblastoma patients, two recurrent solid tumour patients, and three recurrent brain tumour patients. We have successfully produced anti-tumour vaccines for five patients and have treated two patients safely. Our first patient with recurrent osteosarcoma has completed treatment. She received four vaccines, tolerated them very well and now has stable disease; a very good result. The consultants continue to recruit patients. Several patients will soon be at the right stage in their treatment for us to either make or give them their vaccines. We are confident that we will be able to meet our recruitment and scientific goals as outlined above.
Dr Denise Caruso

You must be logged in to post a comment.